German scientists have deciphered one of the key adaptations of early photosynthesis by reconstructing billion-year-old enzymes.
Using various methods, the research team from the Max Planck Institute (MPI) for Terrestrial Microbiology in Marburg, Hesse, Germany successfully resurrected and studied billion-year-old Rubiscos in the lab.
Rubisco, which is probably the most abundant enzyme on the planet, appeared in primordial metabolisms prior to the presence of oxygen on Earth.
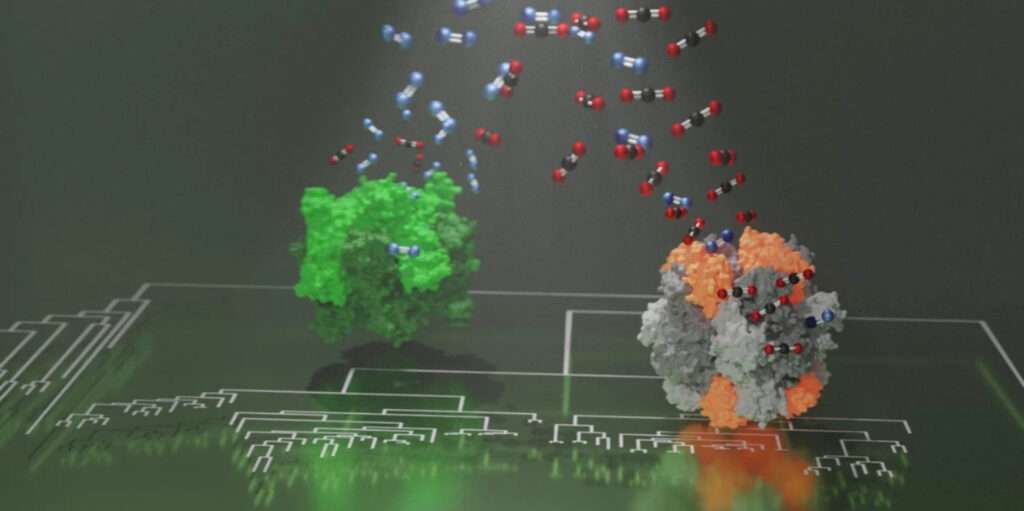
With the emergence of oxygen-producing photosynthesis and rise of oxygen in the atmosphere, the enzyme began mistaking oxygen for carbon dioxide and generated metabolites toxic to the cell.
Even though Rubiscos evolved and became more specific for carbon dioxide over time, none of them could get completely rid of the oxygen-capturing reaction.
But the molecular determinants of Rubisco are still of great interest to researchers, who aim to improve photosynthesis.
This is because those Rubiscos that show increased carbon dioxide specificity recruited a novel protein component of unknown function.
Together with Nanyang Technological University in Singapore, scientists discovered that a completely new component prepared photosynthesis to adjust to rising oxygen levels.
For the purposes of their study, the researchers used a statistical algorithm to recreate forms of Rubiscos that existed before oxygen levels began to rise.
They attempted to solve whether Rubisco’s new component had anything to do with the evolution of higher specificity.
MPI Doctoral Researcher Luca Schulz explained in a statement obtained by Newsflash: “We expected the new component to somehow directly exclude oxygen from Rubisco catalytic center.
“That is not what happened.
“Instead, this new subunit seems to act as a modulator for evolution: recruitment of the subunit changed the effect that subsequent mutations had on Rubisco’s catalytic subunit.
“Previously inconsequential mutations suddenly had a huge effect on specificity when this new component was present.
“It seems that having this new subunit completely changed Rubisco’s evolutionary potential.”
This new function gave scientists answers on why Rubiscos that incorporated the new protein component are completely dependent on it, while other forms can function perfectly well without it.
According to the study, Rubisco becomes tolerant to mutations when attached to the small protein component.
MPI stated: “When bound to this small protein component, Rubisco become tolerant to mutations that would otherwise be catastrophically detrimental.
“With the accumulation of such mutations, Rubisco effectively became addicted to its new subunit.”
Group Leader Georg Hochberg said: “The fact that this connection was not understood until now highlights the importance of evolutionary analysis for understanding the biochemistry that drives life around us.
“The history of biomolecules like Rubisco can teach us so much about why they are the way they are today.
“And there are still so many biochemical phenomena whose evolutionary history we really have no idea about.
So it’s a very exciting time to be an evolutionary biochemist: almost the entire molecular history of the cell is still waiting to be discovered.”
Max Planck Director Tobias Erb stated that the study indicated how photosynthesis might be improved.
He said: “Our research taught us that traditional attempts to improve Rubisco might have been looking in the wrong place: for years, research focused solely on changing amino acids in Rubisco itself to improve it.
“Our work now suggests that adding entirely new protein components to the enzyme could be more productive and may open otherwise impossible evolutionary paths.
“This is uncharted land for enzyme engineering.”
The study was published in the peer-reviewed academic journal ‘Science’ on Thursday, 13th October.



